24 April 2025: Independence, OH – Great Lakes NeuroTechnologies (GLNT) is excited to announce that it has entered into a distribution agreement with Henry Schein Medical UK to distribute GLNT’s Kinesia™ motor assessment system line of products, including Kinesia ONE™, Kinesia 360™, and KinesiaU™, in the UK. This follows the decision by NICE (National Institute for Health and Care Excellence) to include KinesiaU and Kinesia 360 in its evidence-based recommendations for devices for remote monitoring of Parkinson’s disease.
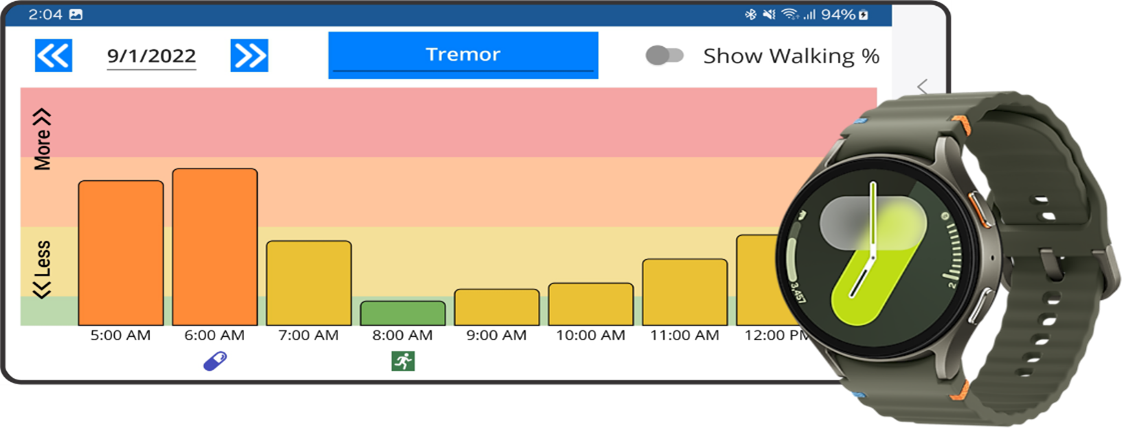
Kinesia systems use proprietary, clinically validated machine learning algorithms based on motion data to monitor motor symptoms such as tremor, slowness, and dyskinesia. User-friendly reports showing symptom severities in response to therapies and activities can help clinicians make better care decisions and identify therapies and activities to improve patient symptoms without requiring an office visit. This partnership with Henry Schein Medical UK will significantly expand access to high-quality objective motor assessment for the over 150,000 people in the UK living with Parkinson’s disease.
To purchase Kinesia products in the UK, please visit Henry Schein Medical UK at https://www.henryschein.co.uk/search/kinesia?type=Products
About Great Lakes NeuroTechnologies
Great Lakes NeuroTechnologies [ www.GLNeuroTech.com ] is committed to pioneering innovative biomedical technologies to serve research, education, and medical communities, improving access to medical technology for diverse populations, and positively impacting quality of life for people around the world.
About Kinesia™ Technology
GLNT commercialized Kinesia™ technology to provide wearable, objective, and automated assessment of movement disorders such as Parkinson’s disease (PD) and essential tremor (ET). The clinically validated technology has been adopted as the gold-standard for objective sensor measurement for movement disorders by many of the world’s leading pharmaceutical and medical device companies. The Kinesia family of motor assessment systems are approved in many regions around the world, but due to individual country regulations, such as language translations, data restrictions, and other regulatory requirements, specific Kinesia products lines may not be approved or available and therefore should not be used in those locations. The Kinesia technology is patented in the US, India, and various countries in Europe including the UK (see https://www.glneurotech.com/patents).
Media Relations
Great Lakes NeuroTechnlogies 1-216-361-5410 media@glneurotech.com
